Seminar: Regioselective Carbometallation of 1,2-Disubstituted Cyclopropenes
June 19th
Seminar Room
9:30
Mr. Yair Cohen (Marek Group)
Regioselective Carbometallation of 1,2-Disubstituted Cyclopropenes
Abstract: Stereodefined poly-substituted cyclopropanes are not only frequent
motifs in biological systems, but also important synthetic building blocks and ideal
substrates for a multitude of selective ring opening reactions. These
transformations lead to valued stereodefined acyclic molecular fragments. As part
of our research campaign to develop efficient routes to polysubstituted three
membered rings as single diastereomers, we recently turned our attention to the
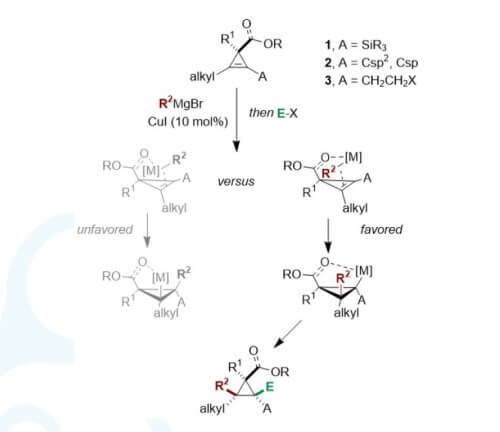
reactivity of 1,2-disubstituted cyclopropenes. We have initially reported the regioand
diastereoselective copper-catalysed carbomagnesiation of two different
activated 1,2-disubstituted cyclopropenyl derivatives, namely cyclopropenylsilanes
and π-substituted cyclopropenes. In both cases, the reaction proceeds smoothly
towards the formation of the electronically favoured regioisomer. For
cyclopropenylsilanes, the stabilized carbanion in the α-position of the silyl group is
preferred, whereas for π-substituted cyclopropenes the favoured regioisomer have
the carbon–magnesium bond conjugated to the π -system. With the aim to further
push the boundary of possible accessible polysubstituted cyclopropane derivatives,
we designed a new approach leading to the formation of a single isomer even for
two electronically unbiased substituents.