-
- A one sentence summary of your research interests.
Research in my lab is focused on biomimetic chemistry and metal coordination aiming to emulate the structure and function of metalloproteins including selective recognition, cooperative catalysis, electro- and photocatalytic water splitting, and self-assembly.
- A more detailed description of your research interests. It should be a few paragraphs in length. Adding a picture or two is a bonus. Keeping the description in the current website is an option if you are happy with it.
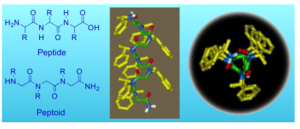
Sequences of a peptoid and a peptide (left) and helical structure of a peptoid bearing chairal bulky phenylethyl groups (right).
We are exploring the interactions between organic biomimetic foldamers (peptide mimics) and inorganic species such as metal ions and metal nanoparticles. Specifically, we are studying peptidomimetic foldamers called “peptoids”, which are N-substituted glycine oligomers.
Peptoids are easily synthesized on solid support by the “submonomer” method – a repetitive two-step protocol in ambient conditions that typically requires short reaction times and no protection groups. Although they are incapable of forming hydrogen bonding along their backbone, peptoids having chiral bulky side chains can adopt secondary structures in solution due to local steric and stereoelectronic interactions.
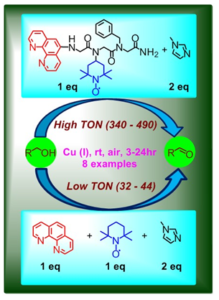
Enhanced activity by an intramolecular cooperative metallopeptoid catalyst.
We are currently involved in five main topics:
-
- Cooperative catalysis. We have recently demonstrated the design of several short peptoids incorporating both a catalytic metal-binding ligand and an organocatalyst, that act in a cooperative manner such that these peptoids could catalyze oxidation of primary alcohols almost 20 times faster than the segregated metal catalyst and organocatalyst. Our results represent the first example of such biomimetic activity by completely synthetic peptidomimetics. We are now developing chiral or helical peptoids in order to make this catalytic transformation asymmetric, aiming for the production of enantiopure alcohols and aldehydes.
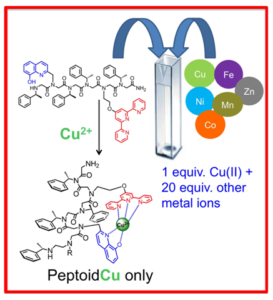
- Selective chelators for Cu(II), Zn(II) and Fe(III). Using helical peptoids and various synthetic techniques for the incorporation of metal-binding ligands with peptoids, we design oligomeric chelators that should have high affinity and selectivity to one metal ion, namely Cu(II) or Zn(II), and two metal ions namely Cu(II) and Fe(III), Zn(II) and Fe(III) and Cu(II) and Zn(II). By incorporating both a N-, O-bidentate ligand and a N-tridentate ligand we have succeeded to generate a peptoid that is highly selective to Cu(II); this peptoid is able to coordinate only Cu(II), in an intramolecular fashion, from a solution mixture containing 20 equiv. of other metal ions e.g. Zn(II), Co(II), Fe(III) and Ni(II). To the best of our knowledge this is the first example of a metal chelator capable of such high selectivity. Moreover, this peptoid could bind two different metal ions, Cu(II) and Fe(III) in an intermolecular fashion, in specific sites along the peptoid by either kinetic or thermodynamic control.
- Microwave assisted chemical reactions on peptoids in the solid phase. We have recently described the incorporation of two bidentate metal-binding ligands via the [3+2] cycloaddition (“click”) reaction under MW irradiation on solid support. In addition, we have shown that we can incorporate an unprotected heterocyclic amine via a substitution reaction between the amine and a propyl chloride attached to the peptoid backbone, done under MW irradiation on solid support. We have also developed an efficient one step cyclization of peptoids under MW irradiation on solid support.
- New biomimetic catalysts for elctrochemical and photochemical water splitting. We are developing water-soluble inorganic clusters as well as peptoids bearing metal-binding ligands and other functional groups, to be used as electro- and photocatalysts for water splitting. We have succeeded to prepare a few complexes that show electrocatalytic activity regarding water splitting and we are now working towards their improvement.
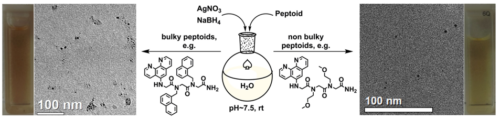
- Metal nanoparticles assemblies mediated by peptoid oligomers. We are designing peptoids capable of binding and stabilizing metal nanoparticles (NPs), and of mediating their aggregation towards applications in chiral sensing and asymmetric heterogeneous catalysis. We have discovered that modifying the peptoid sequence, as well as the NPs binding ligand or the peptoid length has a great effect on the aggregation pattern of the NPs.
Postdoctoral Employment
Aug 2009-Jul 2011 Research with Prof. George Christou, Department of Chemistry,
University of Florida, Gainesville, FL.
Jan 2007-Jul 2009 Research in collaboration with Prof. Michael D. Ward and Prof. Kent
Kirshenbaum, Molecular Design Institute, Department of Chemistry, New York University, New York, NY.
Education
2001-2006 Ph.D. studies in the group of Prof. Ronny Neumann, Department of Organic Chemistry, Weizmann Institute of Science, Rehovot, Israel.
Topic of research: “New Systems for Oxidation Reactions Catalyzed by Polyoxometalates”.
1998-2000 M.Sc. in the group of Prof. Abraham Shanzer, Department of Organic Chemistry, Weizmann Institute of Science, Rehovot, Israel.
Topic of research: “Synthesis, Characterization and Utilization of a New Ligand for the Preparation of Heteronuclear Complexes”
1995-1998 B.Sc. in Chemistry. Tel-Aviv University, Israel (MAGNA CUM LAUDE)
Awards
2011 National Science Foundation (NSF) scholar in Green Chemistry.
2010 Joseph Breen Memorial Fellowship in Green Chemistry (ACS award).
2009 The Stereochemical Society of Greater New York travel award.











