Synthesis and Reactivity of Iron PCNHCP complexes: A Noble Odyssey
June 27th
 חדר סמינרים וזום
חדר סמינרים וזום
11:30
Mr. Subhash Garhwal (de Ruiter Group)
Synthesis and Reactivity of Iron PCNHCP complexes: A Noble Odyssey
Abstract: Transition metal complexes are well known to catalyze various chemical transformations, typically containing a precious metal such as ruthenium, iridium, and palladium. The growing effort to overcome the negative environmental and economic impacts associated with precious metals is of current high interest. Because of the general low toxicity and high availability, first-row transition metals are excellent alternatives to precious metals. Indeed, during the past ten years, various transformations have been developed that involve an earth-abundant metals. However, due to the unpredictable redox behavior of first-row transition metals (e.g., one- vs. two-electron chemistry), there is a high demand to develop robust, strong and rigid ligand scaffolds that can stabilize base metals in variable oxidation states, while facilitating two electron chemistries from a low-spin manifold.
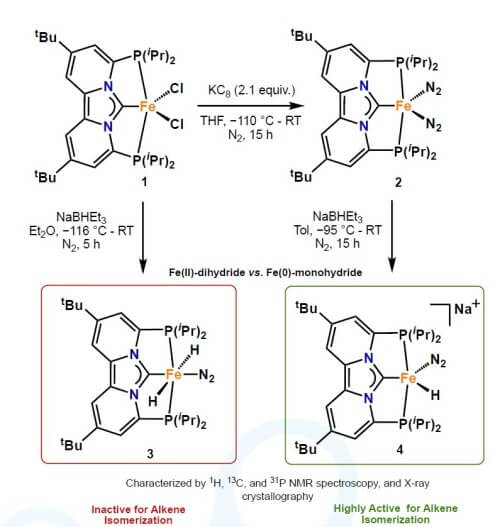
Here we demonstrate that a new pincer type ligand – based on a PCNHCP motif – can stabilize Iron in a low spin state to enable two electron chemistry. Our newly developed pincer ligand can stabilize a highly unusual iron(ii) dihydride pincer complex, the first of its kind that is stable under a dinitrogen atmosphere. This complex was found to be highly effective for hydrogen isotope exchange at (hetero)aromatic hydro-carbons (J. Am. Chem. Soc. 2020, 142, 17131). Furthermore, we will also demonstrate that changing the oxidation- and/or spin-state of these type of metal complexes is important for discovering new reactivity. In particular, we will show that an anionic iron(0) monohydride complex is highly active for alkene isomerization. This newly discovered catalyst is active at the part-per-million level and gives the corresponding 2-alkene in high yield and good stereoselectivity. Most remarkably, the reactivity of these complexes is due to a change in spin-state (singlet to triplet) facilitated by the ligand platform. As a result, there is marked difference in alkene isomerization activity between an iron(ii) dihydride (inactive) and an iron(0) monohydride (highly active), which will be further discussed in this talk Chem Catalysis 2021, DOI: 10.1016/j.checat.2021.05.002.). Beside, iron hydride chemistry, I will also discuss potential utilization of these complexes in variety of other transformations including (i) iron catalyzed aryl-aryl cross-coupling via a two-electron mechanism, (ii) directed C-H bond activation and (iii) functionalization of iron alkylidenes and alkylidynes.

